Our unique aproach
Our unique & versatile HERMES vectors are characterized by their ability to provide:
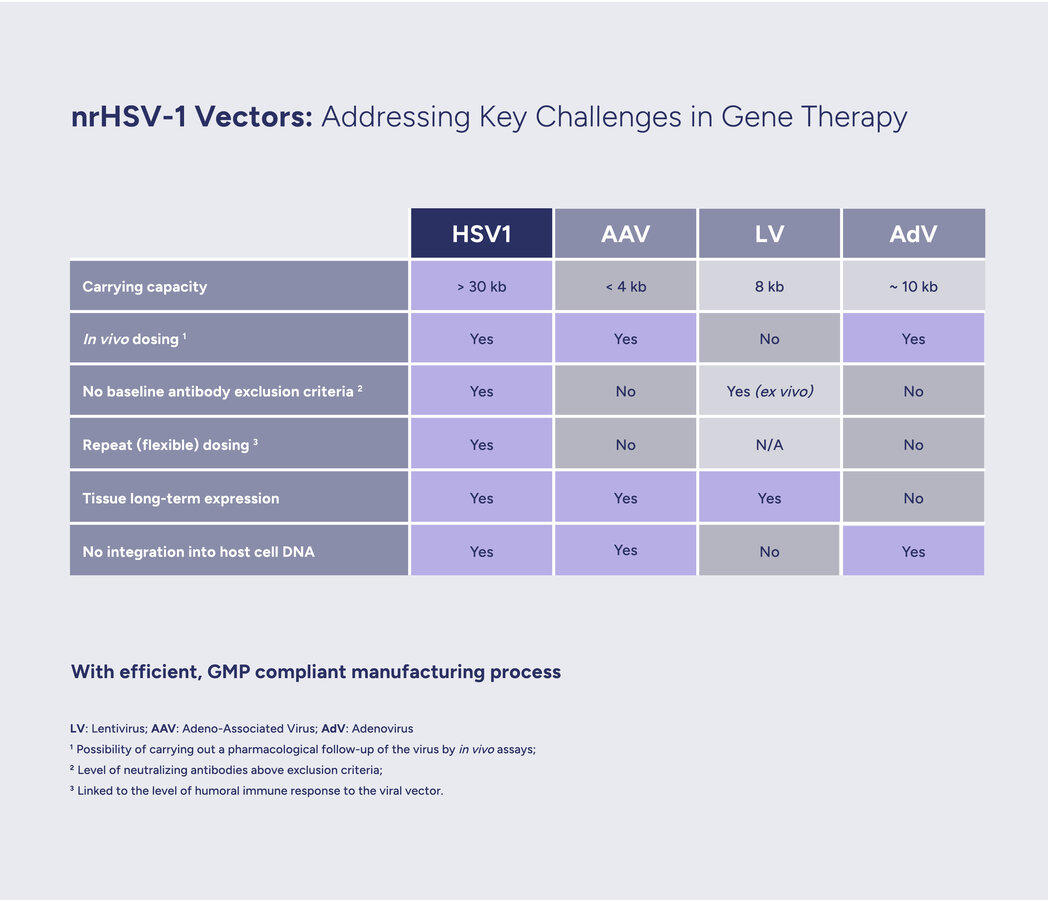
Large capacity. The modified vector can carry a larger genetic payload, of over 30kb, than most other viral vectors (e.g. adeno-associated viruses). The possibility to deliver more in one go enables the inclusion of regulatory sequences to finely tune gene expression, as well as the full genetic instructions for building large or multiple therapeutic proteins.
Long-term expression potential. Leveraging the ability of HSV to reach a long-term latent state, the embedded therapeutical genes can be efficiently expressed over time.
Benign immune profile. All clinical studies of HSV-based vectors, have constantly demonstrated very limited immune response, including the ability to redose in the absence of neutralizing antibodies.
We have developed a uniquely efficient platform from our universal vector and associated manufacturing cell lines. This allows us to go from identification of a potential therapeutic approach to having a vector with full in vitro validation in a few months.
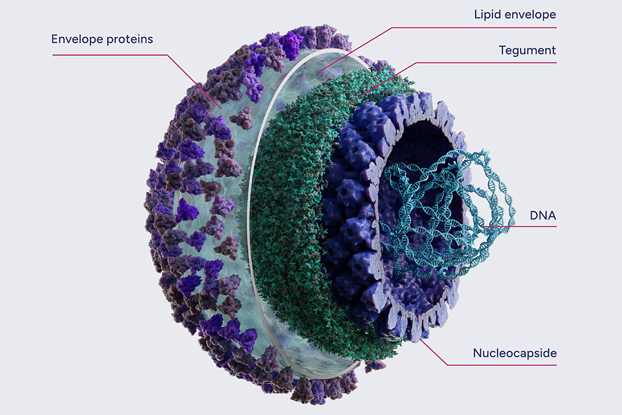
Wild-type HSV
Infection with a herpes simplex virus type 1 (HSV-1) is very common globally. It’s estimated that around 3.7 billion people, 2/3 of the world’s population between the ages of 0 and 49 are infected, although many people never develop symptoms. Once infected, an individual carries the HSV-1 genome for the rest of their life, as it establishes lifelong dormant (‘latent’) infection in the host’s peripheral neurons. During latency, the HSV-1 genome is maintained as an episome, which undergoes only limited gene expression. At EG 427, we used this ability of HSV-1 to establish latency in developing our innovative and powerful therapeutic approach.
Non-Replicative HSV
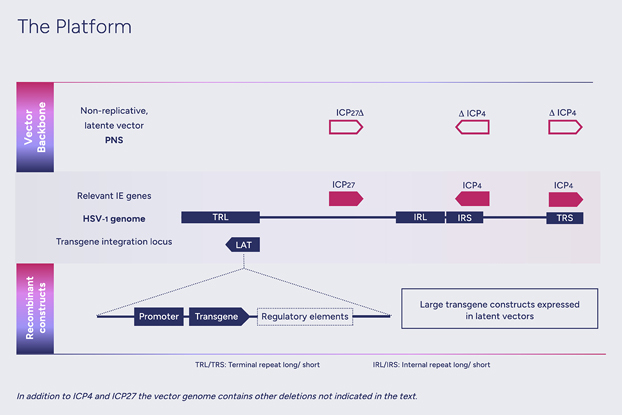
HSV-1 genes can be divided into three groups based on their time of expression: 'immediate early', 'early' and 'late'. Deletion, or inactivation, of immediate early genes prevents the expression of all other viral genes, thus disabling viral replication. These precision deletions are at the heart of our approach to convert HSV-1 into a safe and efficient non-replicative gene therapy vector, which we call nrHSV-1. Moreover, such truncations of the viral DNA make available genomic space within the icosahedral capsid to carry large (over 30kb) genetic cargo.
In the latent state, the Latency-Associated Transcripts (LAT) region remains transcriptionally active, allowing long-term expression of transgene(s) inserted within this region. This is a component of our approach that is fundamental for the treatment of chronic diseases.
Scientists have also demonstrated that the administration of nrHSV-1 does not result in reactivation or recombination events with latent wild-type HSV-1, thus ensuring an excellent safety profile even with a highly prevalent human virus (see white paper).
Manufacturing
Addressing more major chronic diseases requires highly efficient manufacturing processes. Part of our nrHSV-1 platform, we have developed broad expertise and know-how in the manufacturing of our vectors. This includes using unique producer cell lines to address manufacturing productivity and quality challenges. These vital production tools provide the missing viral genes to allow vector production using simple and efficient infection process in our bioreactors. Engineering, maintenance, and improvement, of these cell lines requires considerable technical expertise, which we have in-house.
Our manufacturing processes, both upstream and downstream, have been developed up to GMP-grade, using fully scalable bioreactor processes, to deliver large volume and low cost of goods products, to address major chronic diseases.
Research
By further engineering our vector platform, we are expanding the platform’s capabilities to address unmet clinical need in a growing array of indications beyond PNS. These include CNS neurons, and other non-replicative, or slowly replicative, cell types.
Based on this versatility of our HERMES platform, we are exploring, alone and through our collaborations, different localized, chronic indications.
As product and process are tightly linked, we keep pushing the limits of the manufacturing yields that can be achieved, to deliver higher volumes and lower cost of goods.